Citation
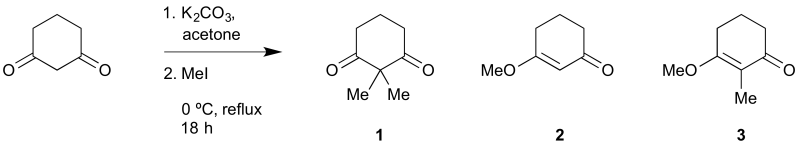
Tritch, K. J.; Brown, C. D.; Shudy, J. E.; Noland, W. E. ChemSpider Synthetic Pages 2017, 813, 1.
Publication URL
Publication Year
Image
Publication Number
3
Image
